Endothermic and exothermic reactions lab answer key – Unveiling the intricacies of endothermic and exothermic reactions, this lab answer key serves as an authoritative guide, meticulously crafted to illuminate the fundamental concepts and practical applications of these intriguing chemical processes. Prepare to delve into a realm where energy exchange governs reactions, shaping our understanding of the world around us.
As we embark on this scientific exploration, we will dissect the defining characteristics of endothermic and exothermic reactions, unraveling their distinct energy dynamics. Through a comprehensive analysis of real-world examples, we will witness the transformative power of these reactions in everyday life.
Additionally, we will equip you with the tools to identify and differentiate between endothermic and exothermic reactions, empowering you to navigate the complexities of chemical reactions with confidence.
Endothermic and Exothermic Reactions
Chemical reactions involve changes in energy. Some reactions absorb energy from their surroundings, while others release energy. These two types of reactions are known as endothermic and exothermic reactions, respectively.
Definitions
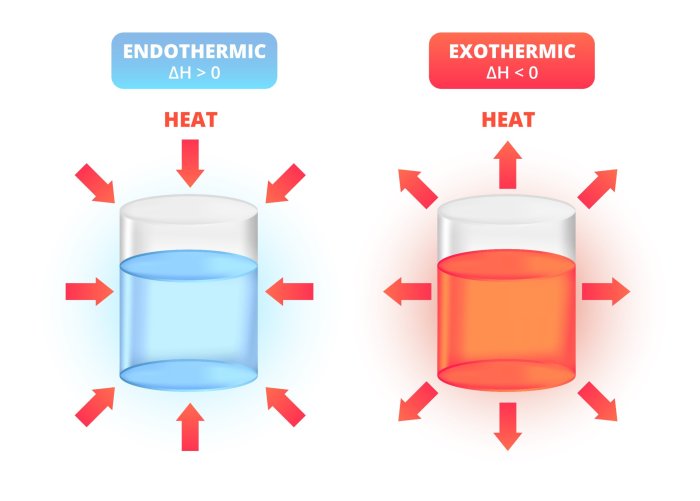
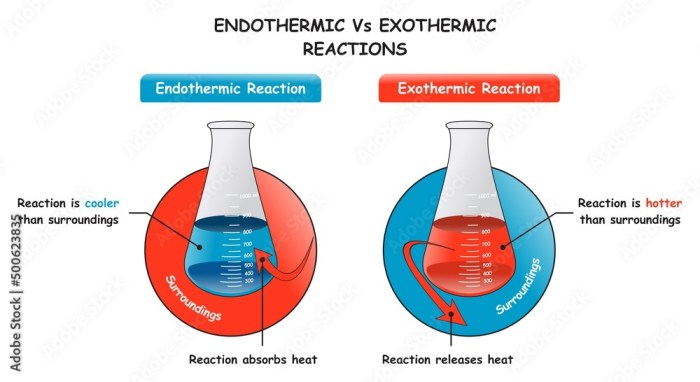
Endothermic Reactions: These reactions absorb energy from their surroundings to form products. The energy absorbed is stored in the products, making them more energy-rich than the reactants.
Exothermic Reactions: In these reactions, energy is released into the surroundings as the products are formed. The products have less energy than the reactants, and the released energy can be observed as heat, light, or sound.
The key difference between endothermic and exothermic reactions is the direction of energy flow. In endothermic reactions, energy flows from the surroundings into the products, while in exothermic reactions, energy flows from the products to the surroundings.
Examples, Endothermic and exothermic reactions lab answer key
Examples of Endothermic Reactions:
- Melting of ice
- Dissolution of ammonium chloride in water
- Photosynthesis
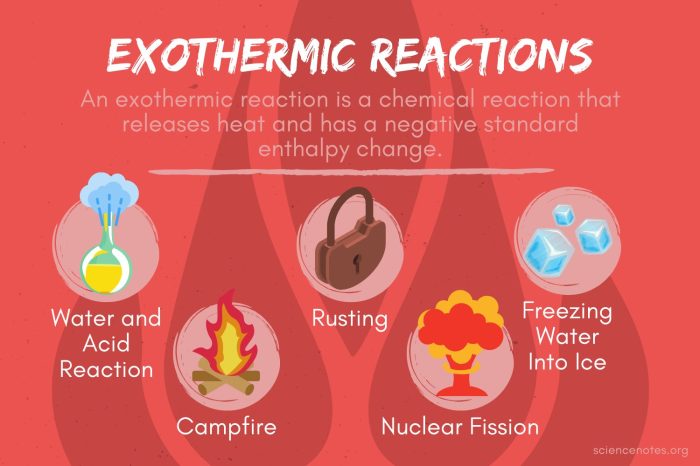
Examples of Exothermic Reactions:
- Combustion of fuels
- Neutralization of acids and bases
- Rusting of iron
| Reaction Type | Energy Change | Examples |
|---|---|---|
| Endothermic | Energy absorbed | Melting of ice, photosynthesis |
| Exothermic | Energy released | Combustion of fuels, neutralization of acids and bases |
Top FAQs: Endothermic And Exothermic Reactions Lab Answer Key
What is the key difference between endothermic and exothermic reactions?
Endothermic reactions absorb energy from their surroundings, while exothermic reactions release energy into their surroundings.
Can you provide an example of an endothermic reaction?
Dissolving ammonium chloride in water is an example of an endothermic reaction.
What is a common application of exothermic reactions?
Exothermic reactions are used in hand warmers and heat packs to generate heat.